Azithromycin and COVID-19
The lack of effective treatments for COVID-19 has spawned an intense, worldwide drug repurposing effort. Although facing steep odds, this approach offers the advantage that candidates will have already undergone human safety assessment and so could be fast-tracked to approval on that basis. The first of these, an unsuccessful trial of the combination HIV therapy lopinavir-ritonavir was recently published. No doubt there will be many more.
Unreviewed reports that certain antimalarial drugs could be effective in treating COVID-19 have circulated for months. Based on still-unpublished work, Chinese authorities have reportedly recommended chloroquine (CQ) as a treatment. On March 19, the US FDA announced that CQ (together with remdesivir) would be placed under Expanded Access (Compassionate Use) provision. This designation permits drugs not approved for a specific indication to be used by doctors outside of a clinical trial.
On March 19, a preliminary report was published detailing the treatment of COVID-19 patients with either hydroxychloroquine (HCQ) alone or in combination with azithromycin (AZ). The paper has since appeared as a pre-proof. There appear to be no discrepancies between the two documents. In this article, I'll focus on an aspect of this study that's received relatively little attention - namely the possibility that AZ itself, without HCQ or CQ, could be an effective treatment against COVID-19.
The Study
The study consisted of a control group of 16 patients who received no drug and a treatment group of 20 patients who received 200 mg oral HCQ sulfate three times daily for ten days. Six patients in the HCQ group additionally received AZ in two doses: 500 mg on Day 1 and 250 mg on Days 2-5. Patients were classified according to whether they suffered from upper respiratory tract infection (URTI) or lower respiratory tract infection (LRTI, confirmed by CT scan).
Six patients in the original treatment group of 26 were dropped (leaving the 20 that were reported). All were receiving HCQ alone. Reasons for halting treatment included:
- three patients, all "PCR-positive," were transferred to the ICU,
- one patient who died "PCR-negative" the day before
- one "PCR-negative" patient who decided to leave the hospital
- one "PCR-positive" patient who stopped treatment due to nausea
Excluding these patients from the treatment group clearly increases increases its apparent response to therapy.
The endpoint of this "ongoing" study was six-day clearance of SARS-CoV-2 RNA in nasopharyngeal swabs, as determined by an apparently as yet unpublished realtime reverse transcription-PCR assay.
The authors themselves note limitations of the study and there are several others, some of which include:
- Non-randomized. The mean age of the control group was 37.3±24.0 years, but the mean age of the treatment group was 51.2±18.7 years. The presence of five patients under the age of 18 in the control group but none in the treatment group accounts for some of this difference. The control group contained four asymptomatic patients, but there were only two in the treatment group. The control and treatment groups were physically located at different facilities, rendering the control group useless to detect quality of care differences.
- Open label. Patients and doctors knew whether drug was administered, and which ones.
- No placebo. The control group received no treatment, rather than a placebo.
- Small study. With only 36 patients, it will be difficult to draw firm conclusions.
- PCR endpoint only. CT scans were not performed, despite having been performed to classify patients at the start of the study.
- No patient received AZ alone.
For further analysis of the study, see:
The endpoint, something called "CT value," (aka Ct) deserves some comment. CT(Cycle Threshold) is the number of cycles needed to show a positive result in the realtime PCR assay. The more cycles required to show a positive result, the greater the viral load.
The CT value cutoff for the study was 35 cycles. Those tests showing no RNA after 35 cycles are reported as negative ("NEG"). The more cycles required to see a signal, the smaller the initial concentration in the sample. For reasons that are not explained in the paper, some realtime PCR results below the CT threshold, mainly in the control group, were reported only as positive ("POS").
Results
HCQ treatment alone led to what the authors claim is a significant reduction of viral load after six days.
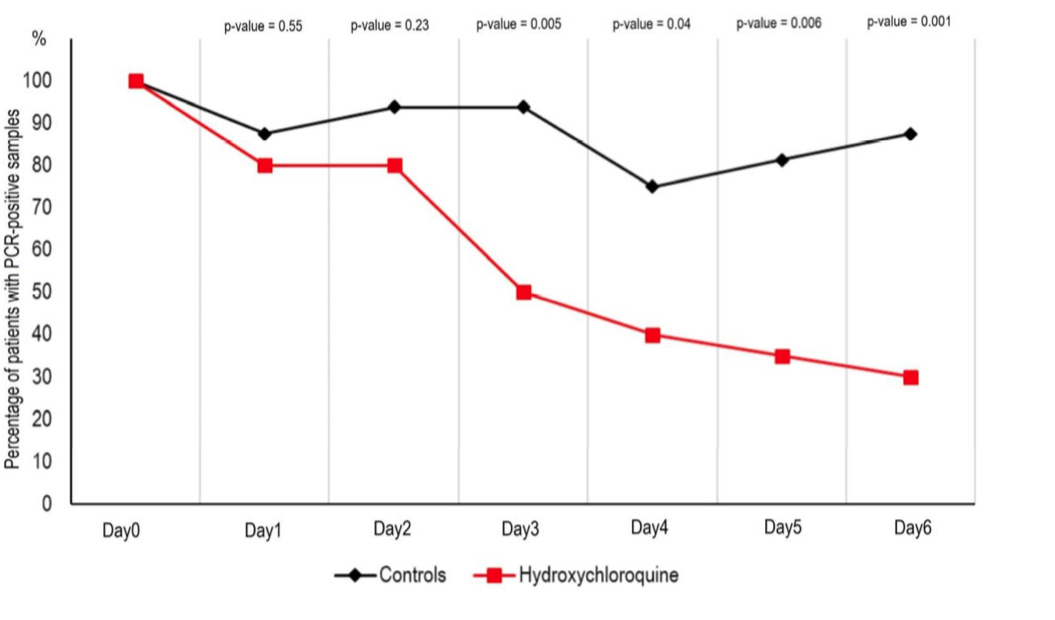
The group receiving HCQ and AZ showed even greater clearance.
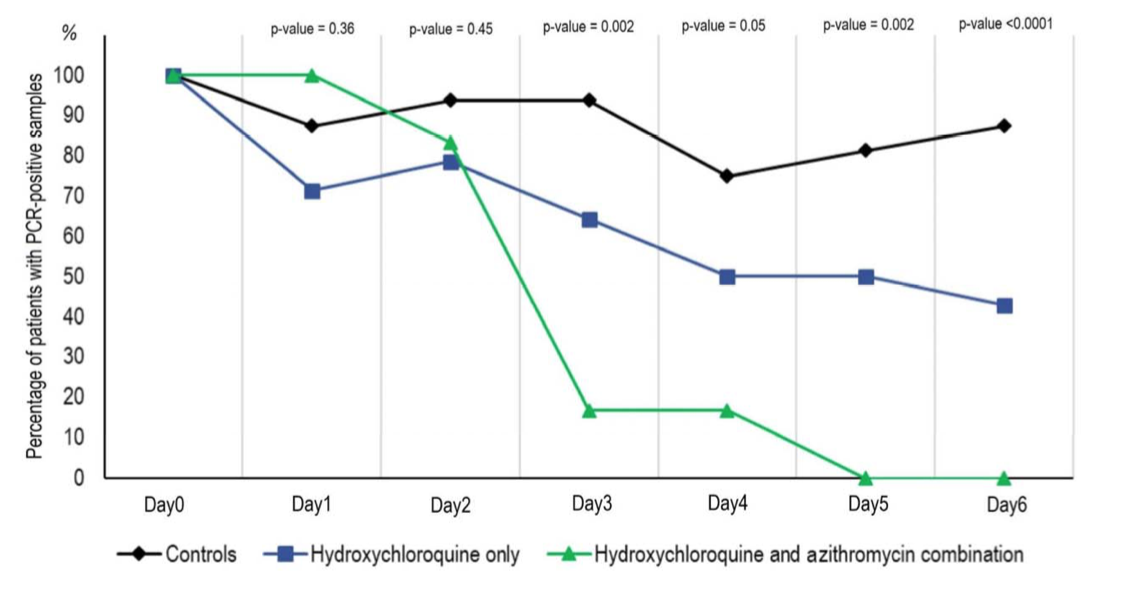
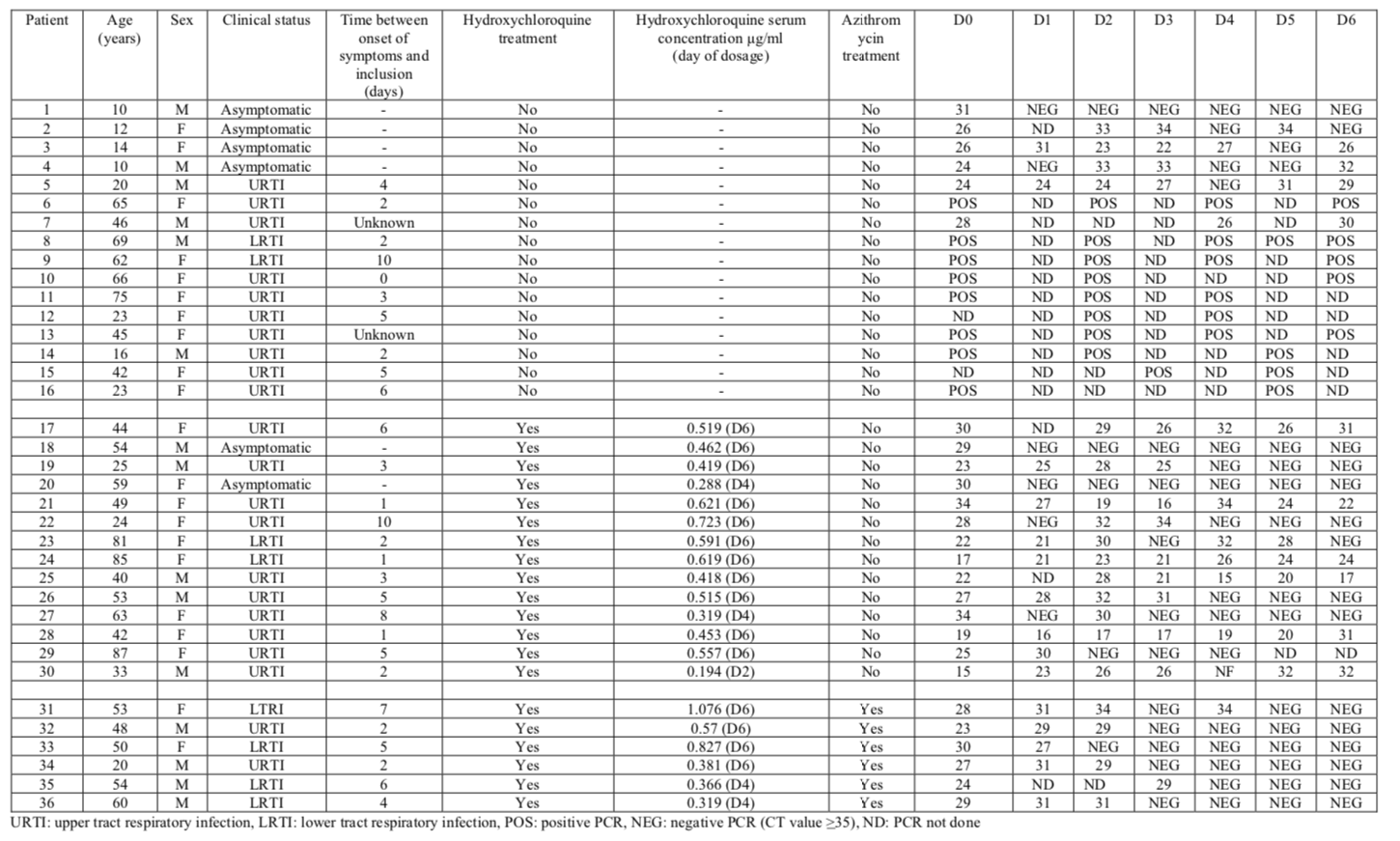
The most useful part of the study is the table, which summarizes all results:
Rationale
Unpublished reports about the efficacy of CQ and HCQ have circulated for weeks now. The in vitro and in vivo antiviral activity of both drugs has been known for some time. For a recent review in the context of COVID-19, see Of chloroquine and COVID-19.
The French study authors note that HCQ's more favorable safety profile as the main reason it was used. A secondary reason was the group's prior experience using the drug to treat Q fever, a bacterial infection.
However, the decision to include AZ in the study seems to have been an afterthought. The authors merely note: "Symptomatic treatment and antibiotics as a measure to prevent bacterial super-infection was provided by investigators based on clinical judgment." In other words, the use of AZ does not appear to have been part of the clinical plan.
The ad-hoc use of AZ may explain why this drug was not administered by itself during the study.
A Striking Result
The French study has problems that will soon be addressed experimentally. Rather than belabor that point, I'd like to focus on the most surprising part of the study:
All of the patients receiving AZ+HCQ recovered, as determined by realtime PCR. Secondarily, some of the HCQ-only patients didn't recover. Even more than that, one of the HCQ-only patients died (and was dropped from the study), and three others were transferred to the ICU (and so were dropped).
The authors note one further anecdote:
Of note, one patient who was still PCR-positive at day6-post inclusion under hydroxychloroquine treatment only, received azithromycin in addition to hydroxychloroquine at day8-post inclusion and cured her infection at day-9 post infection. In contrast, one of the patients under hydroxychloroquine and azithromycin combination who tested negative at day6 post-inclusion was tested positive at low titer at day8 post-inclusion.
To reiterate, these findings come from a flawed study. A lot of work will be needed to sort all of this out. For now, let's consider the role that AZ may be playing in what at this point looks like an uncontrolled, open-label collection of anecdotes.
Synergy?
The authors note that their results "suggest a synergistic effect" for the combination of HCQ and AZ. It's tempting to draw this conclusion, but premature.
The problem is the lack of data for patients treated with AZ alone. Had that trial been conducted, at least three outcomes are possible:
- AZ alone leads to lower viral clearance compared to AZ+HCQ. This would suggest a synergistic effect for the combination, as presumed but not tested by the authors.
- AZ alone leads to no difference in viral clearance compared to AZ+HCQ. This would suggest that HCQ offers no benefit in the treatment.
- AZ alone leads to more rapid viral clearance. This would be consistent with interference of HCQ in treatment by azithromycin, and would suggest that HCQ should be eliminated from the treatment protocol.
Without a clear understanding of the role played by AZ in viral clearance, any future studies using HCQ would seem premature at best. The non-negligible, known side-effect profile of HCQ by itself, and the largely unknown side effects of both medications taken together makes an answer to this question especially important.
Azithromycin as an Antiviral
The authors note:
… Azithromycin has been shown to be active in vitro against Zika and Ebola viruses [citations] and to prevent severe respiratory tract infections when administrated to patients suffering viral infection [citations]. …
Indeed, several scattered studies describing AZ's antiviral activity have been published, including:
- Zika virus cell tropism in the developing human brain and inhibition by azithromycin. AZ was selected from a panel of 2,177 drugs approved for use in pregnancy. This study found "reduced viral proliferation" of Zika virus in an in vitro model.
- Evaluation of Ebola Virus Inhibitors for Drug Repurposing. In a mouse model of ebola, AZ was initially found to increase survival, but this finding could not be repeated. A combination of AZ+CQ was, however, found to be effective.
- Azithromycin Inhibits the Replication of Zika Virus. AZ was found to be effective in a Vero cell viability model.
- Azithromycin induces anti-viral responses in bronchial epithelial cells. In a bronchial epithelial cell model, AZ was found to decrease the rate of replication of rhinovirus. Some mechanistic possibilities were ruled out.
Activity of AZ itself against SARS-CoV-2 is but one of many explanations for the observed viral clearance in the AZ+HCQ group. Others might include: synergy with HCQ; an as-yet unknown effect of AZ on nasopharyngeal sample collection; and tissue-specific effects to name a few.
Conclusion
A small, preliminary COVID-19 clinical study reported complete clearance of virus after combined administration of azithromycin and hydroxychloroquine as measured by realtime PCR of nasopharyngeal swabs. No study of azithromycin alone was conducted. Despite azithromycin's reputation as an antibacterial agent, scattered reports of in vivo and in vitro antiviral activity for this drug have been reported.