Can Your Cheminformatics Tool Do This?
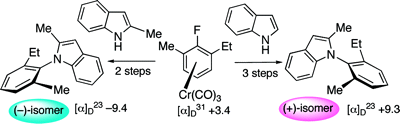
Axial chirality isn't the first thing most chemists come up with when they think of indole. Yet a recent J. Org. Chem. article by Kamikawa et al. describes not only axially chiral indoles, but an enantioselective method for their synthesis.
Axial chirality has been largely ignored by the cheminformatics community. There was once a time when the phenomenon was esoteric enough that it could be reasonably ignored. However, that time has long since passed, as the Kamikawa study, and many others demonstrate.
Several years ago, Andreas Dietz proposed a conceptual framework for solving this problem. More recently, it was put into practice in the FlexMol language and the Octet framework. These may not represent the best solutions to the axial chirality problem, but they clearly demonstrate that a practical solution, fully compatible with modern information technologies, does exists.
Axially chiral molecules like those in the Kamikawa study will increasingly find their way into chemical databases as they continue to become more synthetically accessible. When this happens, users will want to be able to distinguish stereoisomers, just as they wanted (and got) this capability for tetrahedral chirality ten to twenty years ago. When the inevitable request to distinguish axially-chiral stereoisomers in your database comes, how will you respond?